NEWS
-
- 2025.03.31
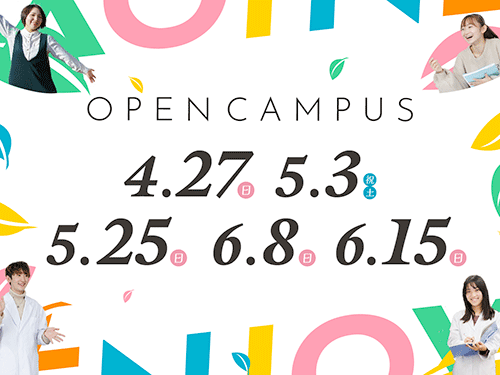
- 2025 オープンキャンパスの申し込みを開始しています
-
- 2025.03.31
- 4月2日(水)入学式のためのスクールバスを運行します
-
- 2025.03.28
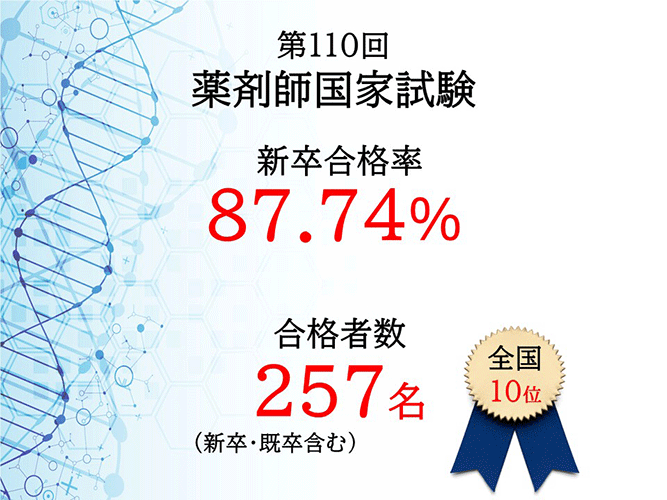
- 第110回薬剤師国家試験の結果について
-
- 2025.02.18
- 学内合同企業説明会を開催しました
-
- 2025.01.21
- 学内合同企業説明会 開催のお知らせ(2月15日オンライン)
-
- 2024.12.27
- 2つの薬局の人事担当者を本学に招いて学内企業説明会(12月分)を開催しました
-
- 2024.10.30
- 3つの薬局の人事担当者を本学に招いて学内企業説明会(10月分)を開催しました
-
- 2025.03.26
- 李宜融教授が「2025台北国際中医薬学術学会」で招待講演を行いました
-
- 2025.03.26
- 第7回高校生サイエンス研究発表会を行いました
-
- 2025.03.26
- 高大連携校の静岡大成高等学校にて出張授業を行いました
-
- 2025.03.26
- 「厚木北地域における防災に関する検討会」に参加しました
-
- 2025.03.25
- 「台湾薬学研修旅行」が開催されました
-
- 2025.03.30
- 2025 オープンキャンパス開催のお知らせ
-
- 2025.03.05
- 会場ガイダンスのお知らせ
-
- 2025.03.31
- 4月2日(水)入学式のためのスクールバスを運行します
-
- 2025.03.26
- 李宜融教授が「2025台北国際中医薬学術学会」で招待講演を行いました
-
- 2025.03.25
- 「台湾薬学研修旅行」が開催されました
-
- 2025.03.31
- 4月2日(水)入学式のためのスクールバスを運行します
-
- 2025.03.28
- 第110回薬剤師国家試験の結果について
-
- 2025.03.25
- 「台湾薬学研修旅行」が開催されました
-
- 2025.02.05
- 第15回卒後教育講座 浜薬漢方セミナーを開催しました
-
- 2024.12.19
- 令和7年2月2日に漢方セミナー(漢方調剤実習)を開催します 参加者募集中
-
- 2024.12.17
- 2024年度の年末年始における窓口業務の休止に関するお知らせ
-
- 2024.11.04
- テレビ神奈川の「猫のひたいほどワイド」で本学の浜薬祭が紹介されました
-
- 2025.03.31
- 2025 オープンキャンパスの申し込みを開始しています
-
- 2025.03.28
- 第110回薬剤師国家試験の結果について
-
- 2025.03.27
- 令和7年「桜並木の一般開放」のお知らせ
-
- 2025.03.24
- 吉江文彦准教授による市民公開講座を行いました
-
- 2025.04.04
- 令和7年度 ハマヤク農園の活動を開始します
-
- 2024.11.26
- 令和6年度のハマヤク農園活動は昨日で終了しました
-
- 2024.11.16
- 2024年11月11日(月)農園日記
-
- 2024.10.31
- 2024年10月31日(木)農園日記
-
- 2024.10.28
- 2024年10月28日(月)ハマヤク農園は中止です
-
- 2025.03.26
- 「厚木北地域における防災に関する検討会」に参加しました
-
- 2025.03.24
- 吉江文彦准教授による市民公開講座を行いました
-
- 2025.03.14
- 本学の準硬式野球部が合同チームで第67回関東地区大学準硬式野球選手権大会に出場しました
-
- 2025.03.31
- 2025 オープンキャンパスの申し込みを開始しています
-
- 2025.04.04
- 令和7年度 ハマヤク農園の活動を開始します
-
- 2025.03.31
- 4月2日(水)入学式のためのスクールバスを運行します
-
- 2025.03.30
- 2025 オープンキャンパス開催のお知らせ
旬な話題をピックアップ!
TOPICS
旬な話題をピックアップ!
7つのキーワ―ドを通して見えてくる"ハマヤク"の魅力